During development of the vertebrate eye, transparent lens structure with refraction power forms to ensure the acquisition of images from the surrounding environment. Lens transparency depends on the degradation of nuclei as well as other organelles in the differentiating lens fiber cells, accompanied by production of large amounts of crystallin proteins to endow the refraction power. Improper regulation of this process may lead to opaque lens, or cataract, which is the leading cause of human blindness. As the denucleation process impedes prolonged transcription of lens specific mRNAs, the massive accumulation of lens specific proteins can only be achieved by highly efficient translation to overcome the progressively compromised lens mRNA production. In this scenario, posttranscriptional mechanisms controlling efficient mRNA translation become especially important, but are not yet clear at the moment.

Figure 1, rbm24a was specifically expressed in the differentiating lens fiber cells
The blue purple signal designates the mRNAs of rbm24a. Le: lens epithelium.
Research from Prof. Shao’s group showed that in zebrafish, the RNA-binding protein Rbm24 is required for the accumulation of crystallin proteins and terminal differentiation of lens fiber cells. In the developing lens, Rbm24 binds to a wide spectrum of lens-specific mRNAs through the RNA recognition motif and interacts with cytoplasmic polyadenylation element-binding protein (Cpeb1b) and cytoplasmic poly(A)-binding protein (Pabpc1l) through the C-terminal region. Loss of Rbm24 reduced the stability of a subset of lens mRNAs encoding heat shock proteins and shortened the poly(A) tail length of crystallin mRNAs encoding lens structural components, thereby preventing their translation into functional proteins. This severely impaired lens transparency and resulted in blindness.
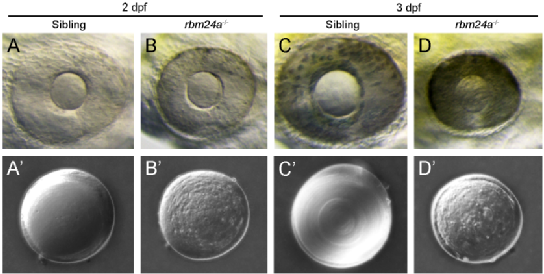
Figure 2, mutation of rbm24a results in opaque lens (cataract)
Consistent with its highly conserved expression in differentiating lens fiber cells, the findings suggested that vertebrate Rbm24 represents a key regulator of cytoplasmic polyadenylation and plays an essential role in the posttranscriptional control of lens development.

Figure 3, Rbm24a controls the translation efficiency of crystallin mRNAs via cytoplasmic polyadenylation mechanism to prevent cataract formation
This work was published in the PNAS on March 13th . Prof. Ming Shao at School of Life Sciences, Shandong University and De-Li Shi at Affiliated Hospital of Guangdong Medical University are co-corresponding authors of this paper. Shandong University is the first author’s organization.